-40%
AutoMedx SAVe Simplified Automated Ventilator 600x10 Portable W/Hard Case
$ 435.57
- Description
- Size Guide
Description
AUTOMEDx SAVe Simplified Automated Ventilator (600 X 10)An automated BVM that delivers an AHA compliant tidal volume of 600 mL at 10 BPM for 5 hours.
This frees up the medic to give compressions, start fluids, treat other injuries or ready the patient for transport.
A single on/off switch eliminates the guesswork and operator error associated with BVMs and overly sophisticated transport ventilators.
Auto
Medx
SAVe
Ventilator
600 x 10
NSN 6515-01-581-8155
Part Number 70000H
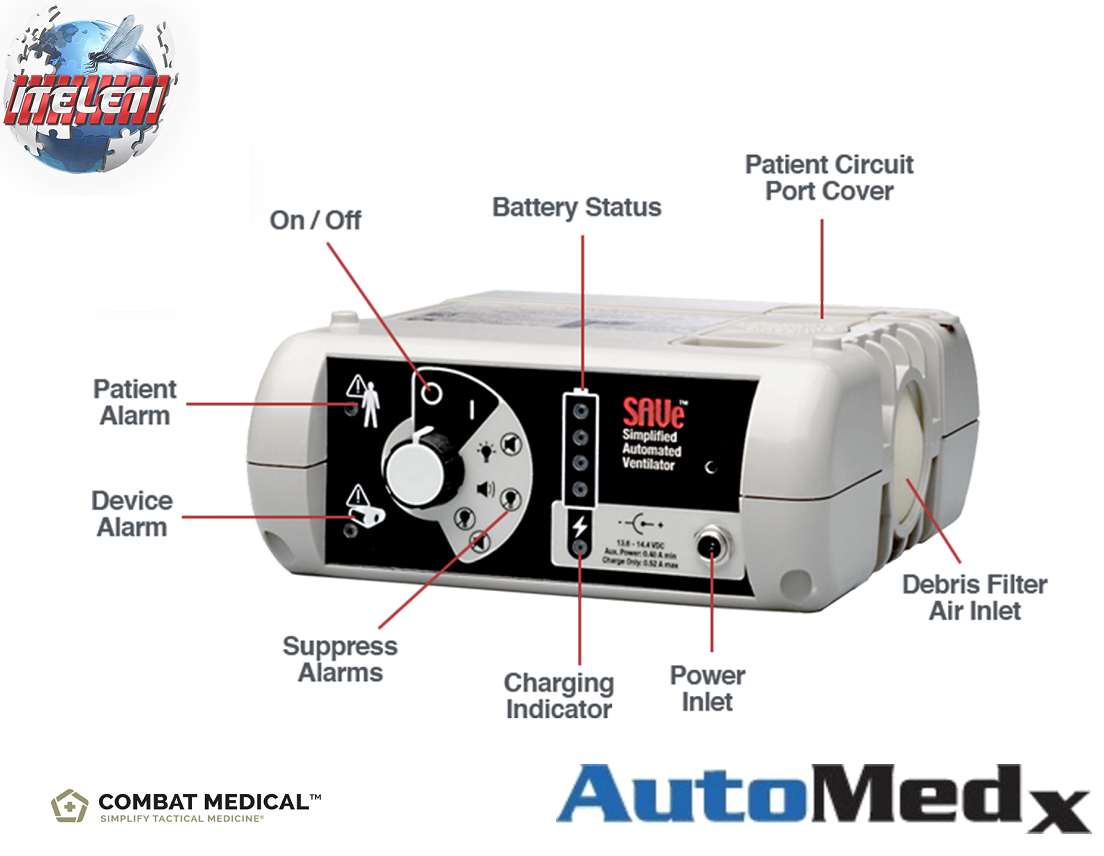
HOW IT WORKS
This listing is for the SAVe Unit and includes only the Accessories listed, as found in the photos.
This Item is being listed as Open Box, sold "AS-IS" but guaranteed to be as pictured and described.
The SAVe Ventilator powers-up and appears to function properly.
We can tell from the DOD Combat Medical Forms that it has been kept in storage and has been handled only to maintain the devices functionality.
Like the Battery should be fully charged after every use. Storing the unit in a discharged state may be detrimental to the internal battery.
Therefore, it is recommended to continue this same routine every 6 months by charging the battery for 14 hours.
INCLUDES:
Simplified Automated Ventilator (SAVe) - Primary Unit
Battery Charger External pow
er supply and AC plug
Oxygen Mask
SAVe Training Slides (Download)
Hard Case (17” X 17” x 13”)
Single-Use Patient Circuit and Pressure Tubing X 2
Supplemental O2 Tubing
Auto
Medx
SAVe
Ventilator
The SAVe uses a rechargeable sealed lead acid battery to drive an
internal pump
that delivers
ambient air for 5 hours per charge.
The device monitors airway pressure and will cut the pump off at 38 cmH2O.
Alarms for disconnect, high pressure and low battery assist the medic in monitoring the patient.
The SAVe does not require O2 to operate but if desired low
pressure supplemental air can be titrated to the patient.
It is a time cycle, volume targeted, pressure limited (38cmH2O) device.
BATTLEFIELD
HOSPITALS
REMOTE
EMS
URBAN
Point of Injury
Intra Hospital Transport
Wilderness
ACLS CPR
Respiratory Failure
CASEVAC
Mass Casualties
Oil Platforms
Backup Ventilator
VIP Security
Battalion Aid Stations
Pan Flu
Ships and Airplanes
Inter Hospital Trans.
EMS Detail
Backup for FST
Crash Cart
Developing Countries
BLS CPR
Rescue
SPECIFICATIONS
TIME CYCLED, VOLUME-TARGETED, PRESSURE-LIMITED
TV:
520-600 ML
BATTERY:
RECHARGEABLE
RR:
10 BPM
DURATION:
5 HOURS
PIP:
38 CMH2O
POWER:
100-240 VAC, 15 VDC,
50-60 HZ
FIO2:
21-62%
SIZE:
6.5” X 6.25” X 2.5”
(102 IN3)
O2:
LOW FLOW SOURCE
WEIGHT:
3.1 LBS | 1.4 KGS
I:E1:
1.7 FIXED
eBay Required - FDA Disclaimer - Any Healthcare Category or Medical Device Listing
The sale of this item MAY be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. This item has been cleaned and handled in accordance with the manufacturer's instructions. If the item was subject to FDA regulation, then we would have to file with the FDA before any verification would commence; such as your status as an authorized purchaser. If you have any questions please contact us or you may refer to the
FDA website:
www.fda.gov
FDA - Medical Devices; Device Regulation and Guidance; Registration and Listing
The following establishments
DO NOT
need to register, list and/or pay fee:
(1)
Import Agent, Broker, and Other Parties who do not take first possession of a device imported into the United States;
(2)
Manufacturer of Components, that are not otherwise classified as a finished device, that are distributed only to a finished device manufacturer (807.65a applies);
(3)
Refurbishers or Remarketers of Used Devices already in Commercial Distribution in the United States;
(4)
Specification Consultant Only;
(5)
Wholesale Distributor that is not a Manufacturer or Importer;
"The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item."
"This Device has been cleaned and handled in accordance with the manufacturer's instructions."